Unveiling the Blueprint for Successful Drug Development: Preclinical and Clinical Considerations for Development

Drug development is a complex and arduous process with high stakes. Bringing a novel therapeutic agent to market requires careful planning, rigorous scientific research, and a thorough understanding of both preclinical and clinical considerations. The book "Preclinical and Clinical Considerations for Development" serves as an invaluable guide through this intricate journey, providing comprehensive insights for researchers, clinicians, and pharmaceutical industry professionals.
Preclinical Considerations
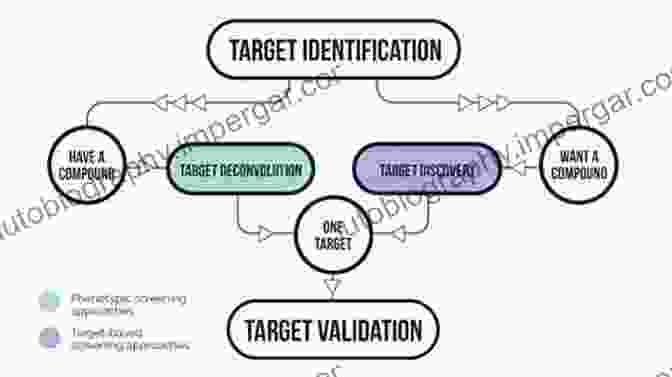
1. Target Identification and Validation
The initial step in drug development involves identifying a molecular target linked to the disease process. This target could be a protein, enzyme, receptor, or other biological entity. Extensive research is conducted to validate the target's role, explore its potential for therapeutic intervention, and ascertain its "druggability."
5 out of 5
| Language | : | English |
| File size | : | 10594 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 551 pages |
| Lending | : | Enabled |
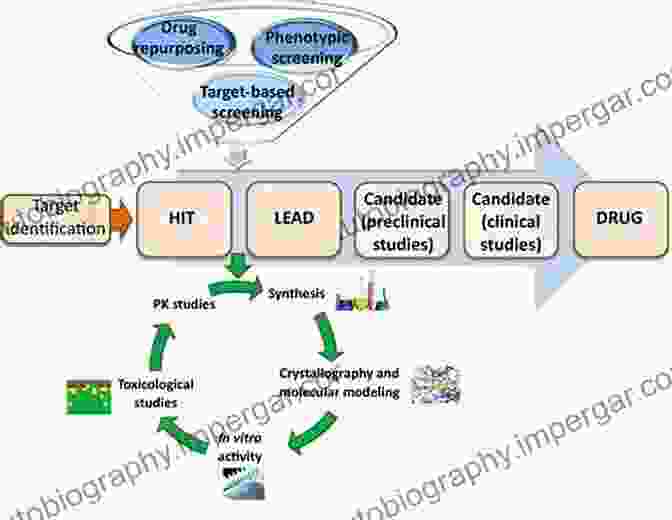
2. Lead Compound Discovery and Optimization
Once the target is established, researchers screen large compound libraries to identify potential lead compounds that bind to and modulate the target. Lead optimization aims to improve the potency, selectivity, stability, and pharmacokinetic properties of the lead compound, transforming it into a drug candidate.
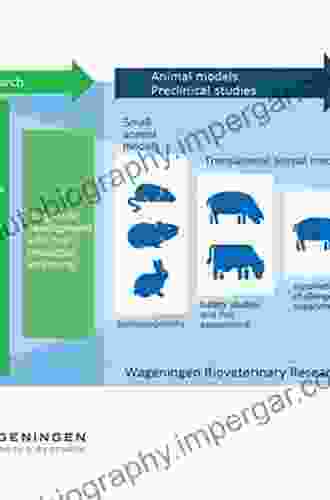
3. Animal Models and Safety Assessment
Animal models play a crucial role in assessing the safety and efficacy of drug candidates. Animal studies help researchers predict potential toxicities, determine appropriate dosing regimens, and evaluate the pharmacological effects of the drug in a living organism.
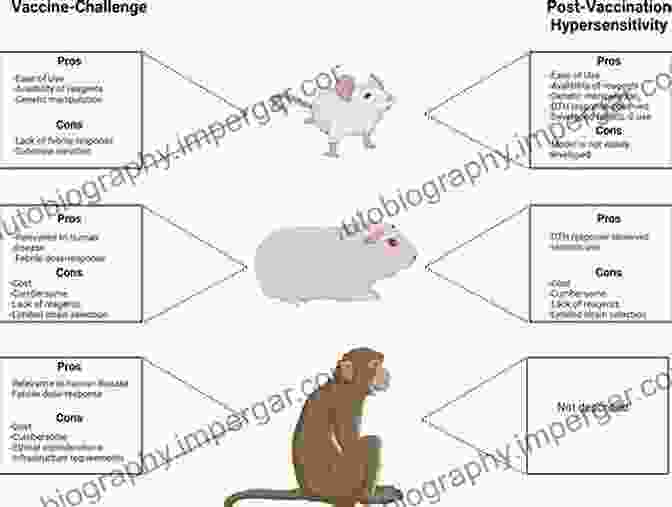
Clinical Considerations
1. Phase I Studies
Phase I clinical trials are the first-in-human studies that assess the safety and tolerability of the drug candidate in healthy volunteers. These studies determine the maximum tolerated dose and identify potential adverse effects. Dose escalation studies aim to establish the optimal dose that balances efficacy and safety.
2. Phase II Studies
Phase II studies expand the clinical investigation to a larger group of patients with the target disease. These trials evaluate the drug's efficacy against a specific endpoint, such as disease progression, tumor shrinkage, or symptom relief. Phase II studies also provide further safety data and identify subpopulations that may benefit most from the drug.
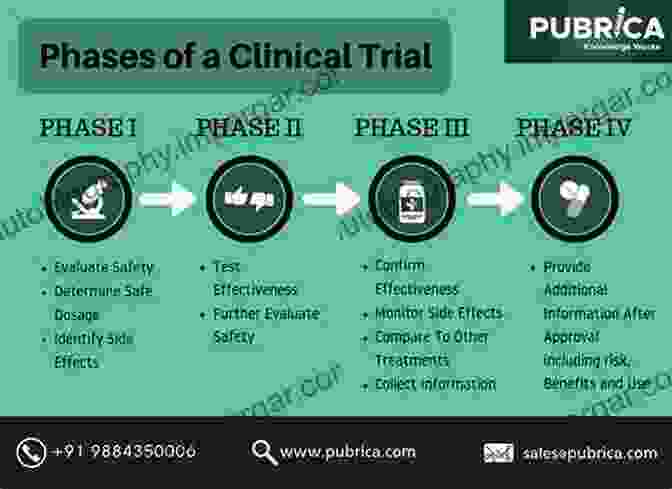
3. Phase III Studies
Phase III studies are large-scale, randomized clinical trials that compare the efficacy and safety of the drug candidate against a standard treatment or placebo. These studies provide definitive evidence of the drug's clinical benefit and establish its risk-to-benefit ratio.
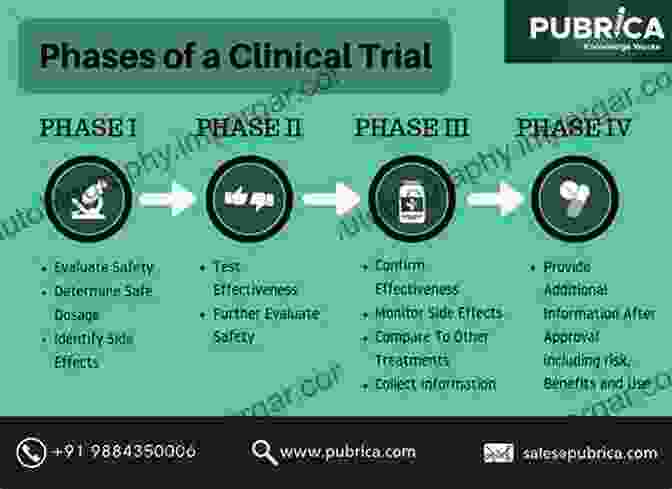
4. Regulatory Approval and Post-Marketing Surveillance
Once the drug successfully completes Phase III studies, it is submitted to regulatory agencies for approval. The regulatory review process involves a thorough assessment of the drug's safety, efficacy, and manufacturing quality. Upon approval, the drug is made available for use in the general population, and post-marketing surveillance continues to monitor the drug's safety and effectiveness in real-world settings.
"Preclinical and Clinical Considerations for Development" is an indispensable resource for anyone involved in the drug development process. Its comprehensive coverage of both preclinical and clinical considerations provides a roadmap for navigating the complexities of drug discovery, optimization, and clinical evaluation. By adhering to the principles outlined in this book, researchers, clinicians, and pharmaceutical industry professionals can increase the probability of successful drug development, ultimately bringing novel therapies to patients in need.
5 out of 5
| Language | : | English |
| File size | : | 10594 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 551 pages |
| Lending | : | Enabled |
Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
 Book
Book Novel
Novel Page
Page Chapter
Chapter Text
Text Story
Story Genre
Genre Reader
Reader Library
Library Paperback
Paperback E-book
E-book Magazine
Magazine Newspaper
Newspaper Paragraph
Paragraph Sentence
Sentence Bookmark
Bookmark Shelf
Shelf Glossary
Glossary Bibliography
Bibliography Foreword
Foreword Preface
Preface Synopsis
Synopsis Annotation
Annotation Footnote
Footnote Manuscript
Manuscript Scroll
Scroll Codex
Codex Tome
Tome Bestseller
Bestseller Classics
Classics Library card
Library card Narrative
Narrative Biography
Biography Autobiography
Autobiography Memoir
Memoir Reference
Reference Encyclopedia
Encyclopedia Christine M Mahoney
Christine M Mahoney Andrew C Isenberg
Andrew C Isenberg J C Sum
J C Sum Melissa R Klapper
Melissa R Klapper Lisa Edwards
Lisa Edwards Sean Daly
Sean Daly Rob Preece
Rob Preece Susan Moore
Susan Moore Don Mayer
Don Mayer Karen Mccall
Karen Mccall Charles Jordan Tabb
Charles Jordan Tabb Martin Sheen
Martin Sheen Ai Weiwei
Ai Weiwei Giorgio Lando
Giorgio Lando William Taubman
William Taubman Trevor Burnard
Trevor Burnard Nilton Bonder
Nilton Bonder Harper Daniels
Harper Daniels Kent Greenfield
Kent Greenfield Joseph Tenenbaum
Joseph Tenenbaum
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Avery SimmonsClinical Lessons on Life and Madness: Unlocking the Secrets of Mental Health
Avery SimmonsClinical Lessons on Life and Madness: Unlocking the Secrets of Mental Health
 Raymond ChandlerUnveiling the Enigma: The History of the Ancient Indo-European Nomads in the...
Raymond ChandlerUnveiling the Enigma: The History of the Ancient Indo-European Nomads in the...
 Patrick RothfussPoromechanics: Unveiling the Secrets of Fluid-Solid Interaction in Porous...
Patrick RothfussPoromechanics: Unveiling the Secrets of Fluid-Solid Interaction in Porous... Gavin MitchellFollow ·5.4k
Gavin MitchellFollow ·5.4k Ezekiel CoxFollow ·14.6k
Ezekiel CoxFollow ·14.6k Reed MitchellFollow ·13.4k
Reed MitchellFollow ·13.4k Stuart BlairFollow ·13.3k
Stuart BlairFollow ·13.3k Harvey BellFollow ·5.4k
Harvey BellFollow ·5.4k J.D. SalingerFollow ·7.3k
J.D. SalingerFollow ·7.3k Jamal BlairFollow ·2.5k
Jamal BlairFollow ·2.5k Frank MitchellFollow ·8.3k
Frank MitchellFollow ·8.3k

 Phil Foster
Phil FosterBookkeeping Essentials: How to Succeed as a Bookkeeper
Bookkeeping is the process...

 Charles Bukowski
Charles BukowskiUnveiling the Unseen: The Occupiers Experience - A...
In the vibrant tapestry of contemporary...
5 out of 5
| Language | : | English |
| File size | : | 10594 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Print length | : | 551 pages |
| Lending | : | Enabled |